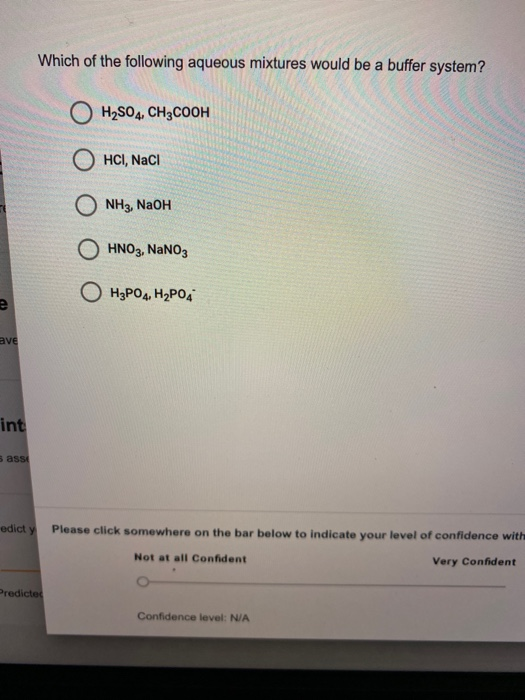
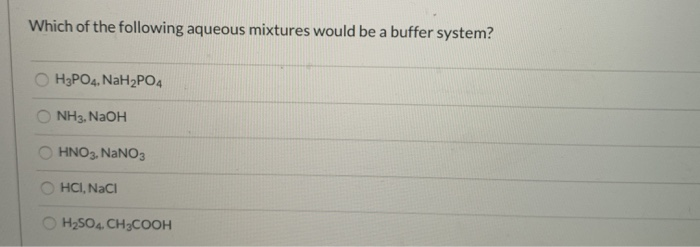
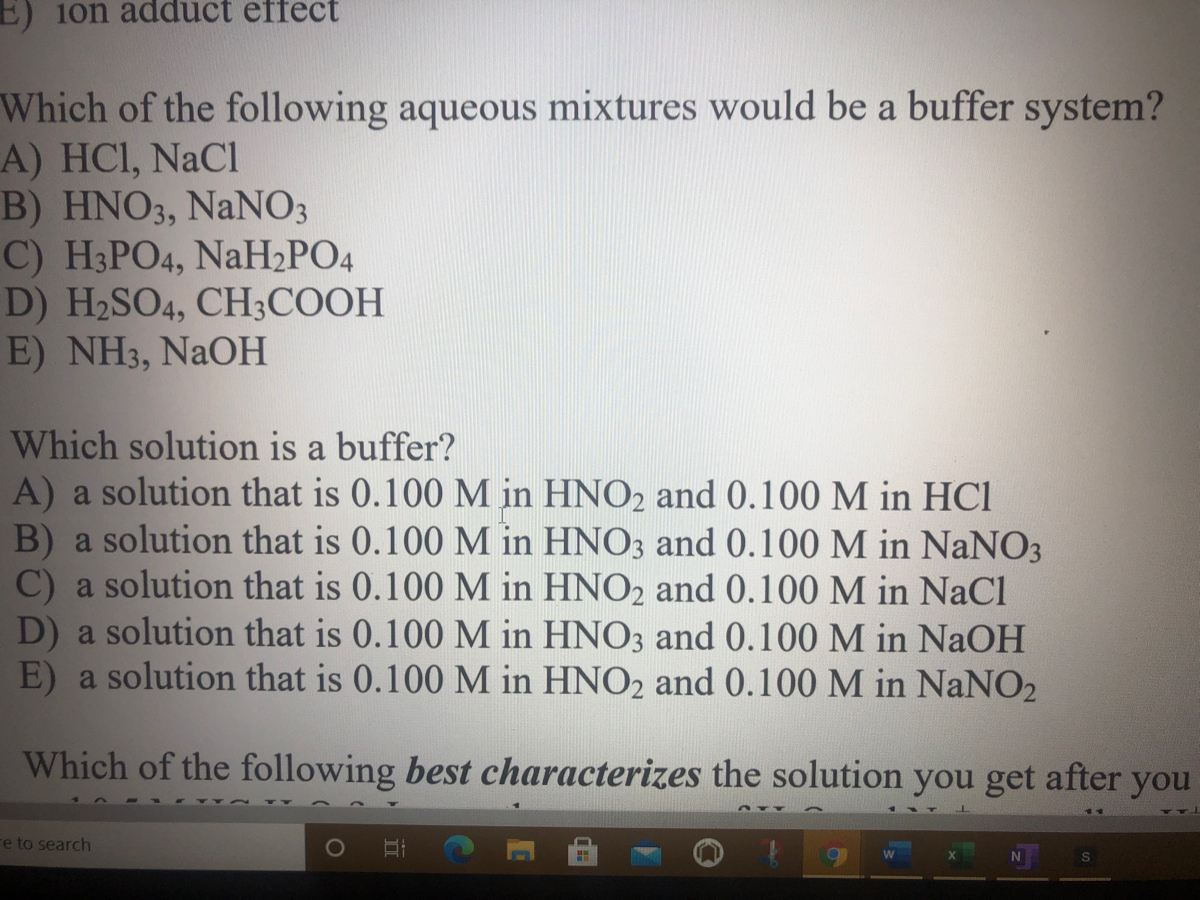
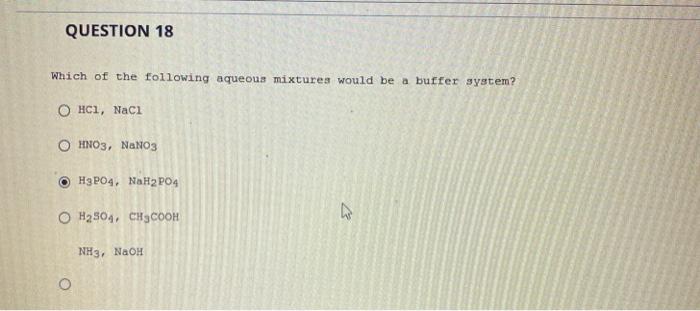
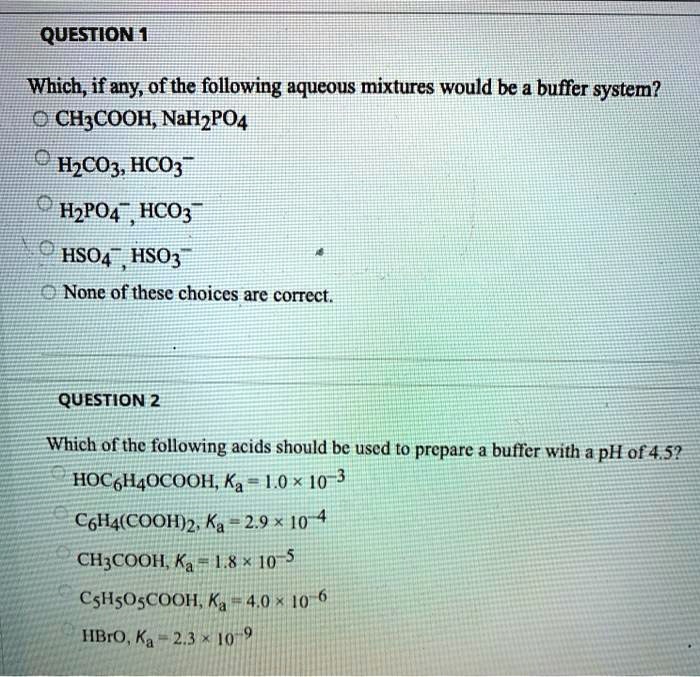
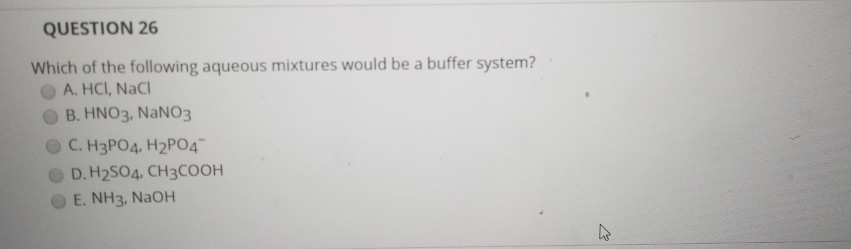
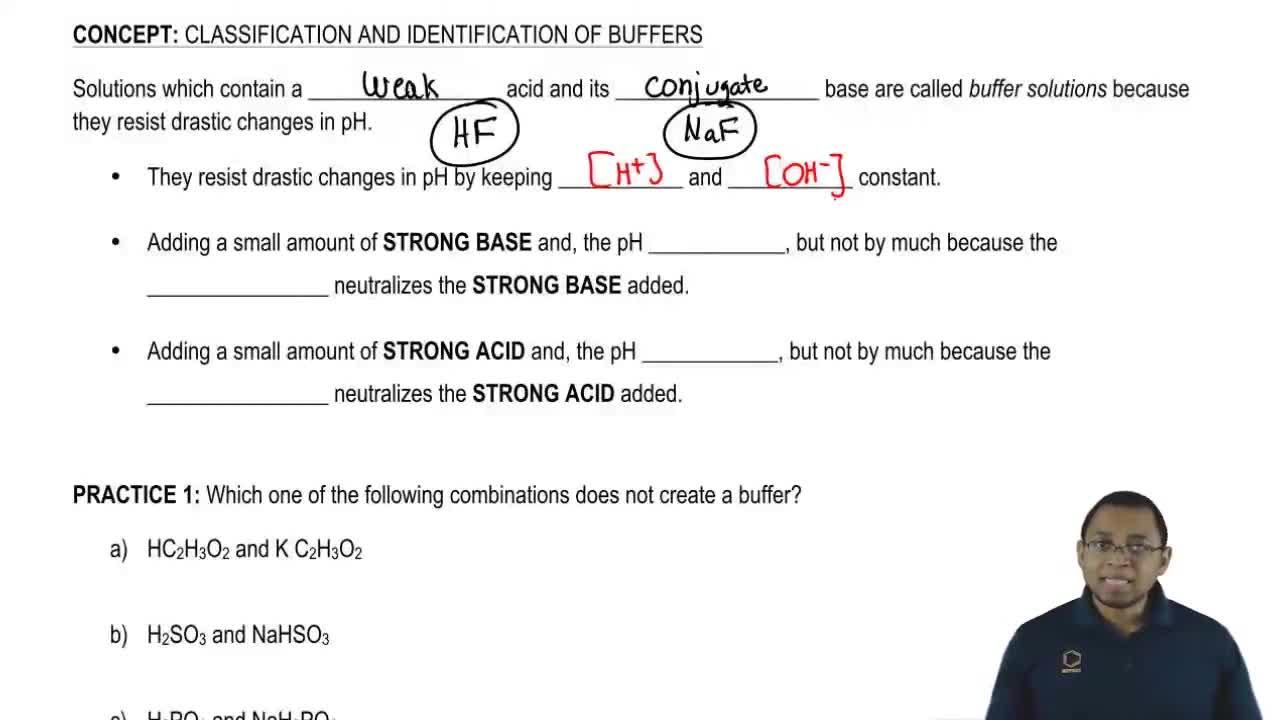
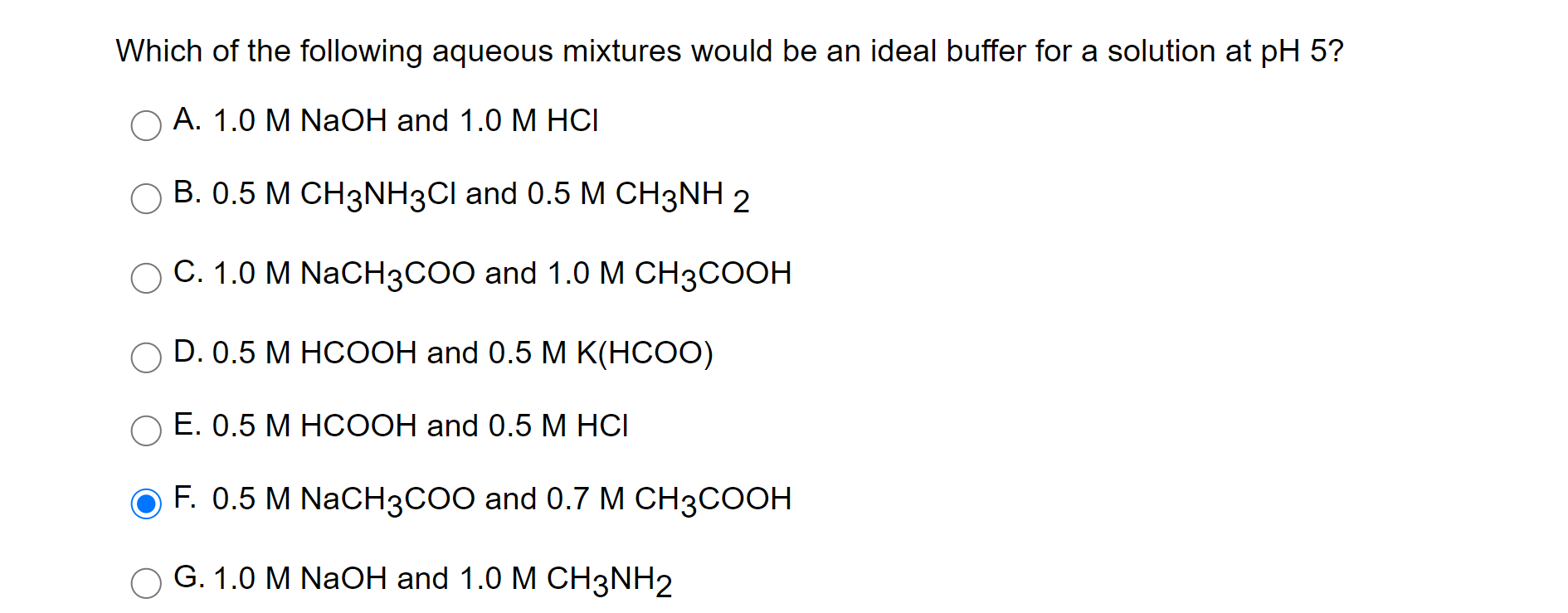
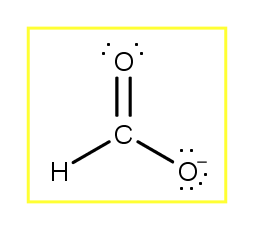
Which Of The Following Aqueous Mixtures Would Be A Buffer System?
Which of the following aqueous mixtures would be a buffer system?. Available both in print and online the Handbook covers 390 chemistry physics and related subjects organized in easy-to-find well. When you are done the system will automatically calculate for you the amount you are expected to pay for your order depending on the details you give such as subject area number of pages urgency and academic level. Single-cell isolation is the first step for obtaining transcriptome information from an individual cell.
A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versaIts pH changes very little when a small amount of strong acid or base is added to it. Turbidimetry UV-VIS spectrophotometry tensiometry electrophoretic mobility measurements SEM. After filling out the order form you fill in the sign up details.
Instrumentation of HPLC As shown in the schematic diagram in Figure above HPLC instrumentation includes a pump injector column detector and integrator or. Today more than ever the CRC Handbook of Chemistry and Physics is critical in ensuring that researchers educators and students have the highest quality data for chemical compounds and physical particles. The following techniques were used.
Add 100g vitamin-free casein Research Organics 765g NaCl 0724g Na 2 HPO 4 anhydrous 021g KH 2 PO 4 to 900 ml H 2 O and 3 ml of 1 M NaOH. A buffer system of acetic acid and sodium acetate works best if. 1441 Drug Release Test Methods for Enteric Coated Products.
Doctor of Philosophy PhD University of Iowa. Michelle Long Yisheng Chen in Developing Solid Oral Dosage Forms 2009. The mobile phase is an aqueous buffer where both pH and ionic strength are used to control elution time.
Colloids also known as colloidal solutions or colloidal systems are mixtures in which microscopically dispersed insoluble particles of one substance are suspended in another substance. Heat with stirring to 80. Gel filtration chromatography a type of size exclusion chromatography can be used to either fractionate molecules and complexes in a sample into fractions with a particular size range to remove all molecules larger than a particular size from the sample or a combination of both operations.
In summary several studies 102029 have shown that commercially available glutaraldehyde represents multicomponent mixtures but knowing which of these components is the most efficient for reactions with proteins is debatable. For such experiments an ionic buffer solution is incorporated in a solid matrix layer composed of paper or a crosslinked gelatin-like substance.
The buffer would adjust the pH in the range of pH 79.
In fact in aqueous solution glutaraldehyde can exist in its simplest form a monomeric dialdehyde but also as. The signal at 190260-nm wavelength was collected. Instrumentation of HPLC As shown in the schematic diagram in Figure above HPLC instrumentation includes a pump injector column detector and integrator or. Ensure you request for assistant if you cant find the section. B large amounts of acid or base are added to the buffered system c the. The mobile phase is an aqueous buffer where both pH and ionic strength are used to control elution time. A suitable system consists of a potentiometer a glass electrode and a reference electrode. Gel filtration chromatography a type of size exclusion chromatography can be used to either fractionate molecules and complexes in a sample into fractions with a particular size range to remove all molecules larger than a particular size from the sample or a combination of both operations. We dont juggle when it comes to pricing.
Make a revision and communicate with your writer exactly what you want adjusted or improved on your paper. The influence of the pseudoamphoteric zwitterionic surfactant cocamidopropylbetaine CAPB on the stabilizing flocculating properties of the aqueous suspensions of glauconite GT with cationic guar gum CGG at various pH values was investigated. The following techniques were used. Turbidimetry UV-VIS spectrophotometry tensiometry electrophoretic mobility measurements SEM. Futami J Fujiyama H Kinoshita R Nonomura H Honjo T et al. Today more than ever the CRC Handbook of Chemistry and Physics is critical in ensuring that researchers educators and students have the highest quality data for chemical compounds and physical particles. A precise pH determination can be made by making an electromotive force emf measurement of a standard buffer solution whose pH is known and then comparing that measurement to an emf measurement of a sample of the solution to be tested.

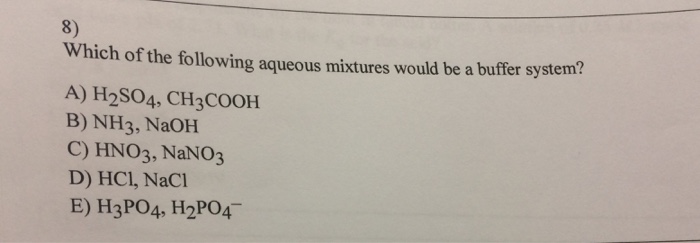


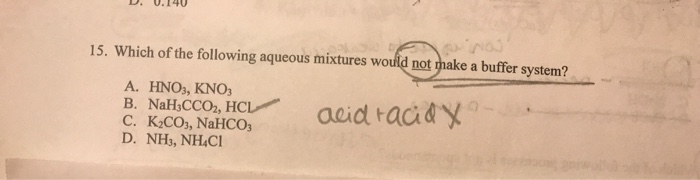








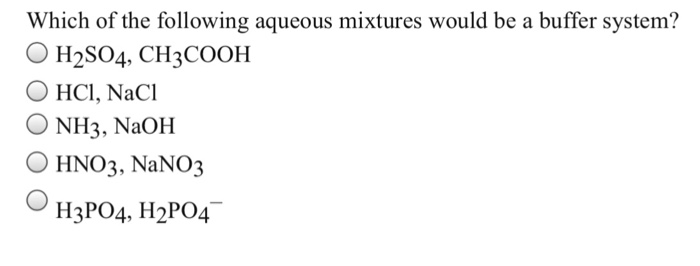


















Post a Comment for "Which Of The Following Aqueous Mixtures Would Be A Buffer System?"